Is this method converting the heat entropy into a coherent light, or in other words, useful work? Sounds like reversing the second law of thermodynamics.
The researchers […] took advantage of small amounts of excess heat to emit more power than consumed. This heat arises from vibrations in the device’s atomic lattice, which occur due to entropy.
Basically yes.
But… this article is 13 years old.
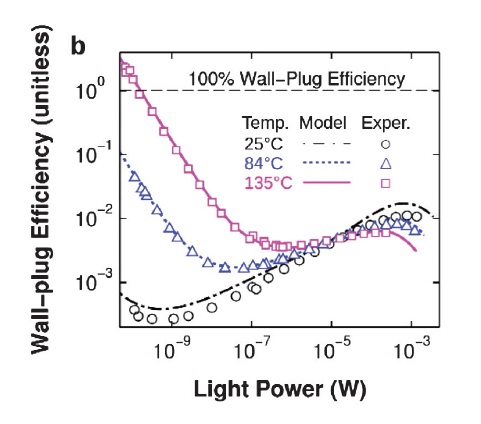
Note how the efficiency sharply drops with a lower temperature; this looks a lot like a heat pump, that we know to not violate the second law.
Based on that my guess is that the loss of local entropy causes a raise of the entropy elsewhere. Probably whatever you’re using to keep the LED warm.
I’ve got new blue LED bulbs that emit 133 lux while consuming 5 watts of electricity, which is 26.6 lumens/watt.
It is so bright that it blows the room out with blue color/light, so I completely believe this article.